Which Statement Best Describes the Ph of Pure Water
B Ionic bonds are very weakmuch weaker than. It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions.

Naturally European Rose Petal Luxury Hand Cream Boxed 20 Shea Butter 75ml You Can Get Additional Details Hand Cream Natural Hand Cream Shea Butter Hand Cream
Transfer sample to a beaker.

. It is basic because it has a hydroxide ion. Boxes Y and W. O H2O is an acid and Cl- is its conjugate base.
9 A given solution has a pH of 10. H2O H OH- Kw 42010-14 at 45oC Kw is ionic product of watertherefore Kw H ˣ. It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions.
It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions. Question 10 1 pts Calculate the pH of pure water at 45 C Kw 420 x 1014 at 45 C. Water molecules are magnetic d.
It is neutral because the concentration of hydronium ions equals that of hydroxide ions. You might be interested in I need help pls asap ty erastovalidia 21 Answer. Sodium hydroxide a compound commonly found in drain cleaners has a pH of 13.
Water molecules are attracted to each other d. Advertisement Answer 50 5 0 mcannady4. Anything higher than 7 would make water acidic already.
The answer is letter A. In a sample of pure water only one of the following statements is always true at all conditions of temperature and pressure. It is acidic because it has a hydronium ion concentration of mc027-1jpg.
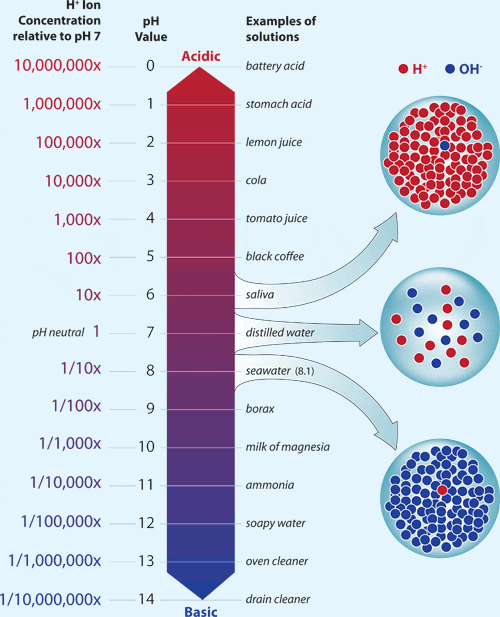
How many molecules of water are there in 2500g water. The diagram below shows a pH scale with several common tasks. Sun Mar 20 2016 The statement that best describes the pH of pure water is it is neutral because the concentration of hydronium ions equals that of hydroxide ions.
You might be interested in. Calibrate the pH meter using pH 7 and pH 4 standardization buffers. Which of these statements best describes the contents of the pile.
H acceptor B Acidic. Cut meat sample into small pieces and weight approximatley 10 grams into a blender cup. Comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms.
Which best explains this. Which statement best describes the ph of pure water. Which statement best describes the pH of pure water.
1 point O HCl is an acid and Cl- is its conjugate base. It is acidic because it has a hydronium ion concentration of mc027-1jpg. Chemistry 22062019 0730 10040813.
NaOH is added to a beaker containing distilled water. 10 Which of the statements below accurately describe ionic bonds. It is neutral because the concentration of hydronium ions equals that of hydroxide ions.
Which of the following best describes that solution. 5 pts Question 7 OH 1 x 10 M OH. The pH of the solution is to the third decimal place Is this solution acidic neutral or basic.
Which statement best describes ph of pure water Other questions on the subject. Boxes X and Z. Water when it is pure has a PH of 7.
Read the pH as soon as possible. Hence the best statement for the pH of pure water is It is neutral because the concentration of hydronium ions equals that of hydroxide ions. Water molecules are wet.
Answer Expert Verified 10 5 5 Hagrid Pure water has a 614 pH in temperatures that are at 100 degrees celsius. HCI H2O Cl H30 Which statement about the reaction is correct. ASHA 777 7 1 year ago 3 0 Pure water has a 614 pH in temperatures that are at 100 degrees celsius.
Use the reaction to answer the question. A H 3 O 10 x 10 -7 M b OH - 10 x 10 -7 M c pH 70 d pOH 70 e H 3 O OH - 5. Anything higher than 7 would make water acidic already.
It is neutral because the concentration of hydronium ions equals that of hydroxide ions. Add 100 ml of distilled deionized water. HW06_ pH Intro opens 2_3-solutionspdf.
It is basic because it has a hydroxide ion concentration of. This just means that pure water is in the neutral zone of the pH scale. They immediately Ine and become one drop.
Which one is always true. It is acidic because it has a hydronium ion concentration of mc027-1jpg. O Acids are substances that contain more protons than bases.
Which of the following statements BEST describes a homogenous solution. When you bring two drops of water near each other and allow them to touch. View the full answer Transcribed image text.
When the value of pH is equal to 7 then the solution is neutral. O Acids are substances that contain fewer protons than bases. The lower the pH the more acidic a compound is.
Water molecules are attracted to each other c. Which of the above figures BEST represents a pure substance. This just means that pure water is in the neutral zone of the pH scale.
The pH of water is 7 which is equal to 10-7 of H and OH- ions. The pH scale shows how acidic or how basic these compounds are. Which statement best describes this solution.
Which statement best describes the ph of pure water. The higher the pH the more basic it is. If water reaches a 7 pH scale it is considered as an alkaline water.
A In ionic bonding atoms share electrons. - 1683462 lykamea99 lykamea99 30072018 Chemistry. If K w is 29 x 10 -15 at 10 o C what is the pH of pure water at 10 o C.
PH is equal to the negative log of hydrogen. Which of the following best classifies pure water and pure sodium chloride NaCl from CHEM 302 at University of Texas. Well-known substance with PH 7 but there are many others most of which are water-based solutions which have a PH of around 7.
Water molecules are made of atom b. Convection is the transfer of heat energy in a fluid. If water reaches a 7 pH scale it is considered as an alkaline water.
Blend for 30 seconds on high speed. The table compares the number of electrons in two unknown neutral atoms. Which statement below BEST describes the changes that occur after the addition of NaOH.
Run duplicates on each sample.

Klar Seifen Inc Video Video In 2021 Natural Soaps Recipes Palm Oil Free Soap Natural Cosmetics Diy

Moda Fabrics The Collector Quilt Pattern Novelty For Quilting Quilt Patterns Mini Quilt Patterns Quilts

Acids And Bases Ck 12 Foundation

17 4 Titrations And Ph Curves Chemistry Libretexts
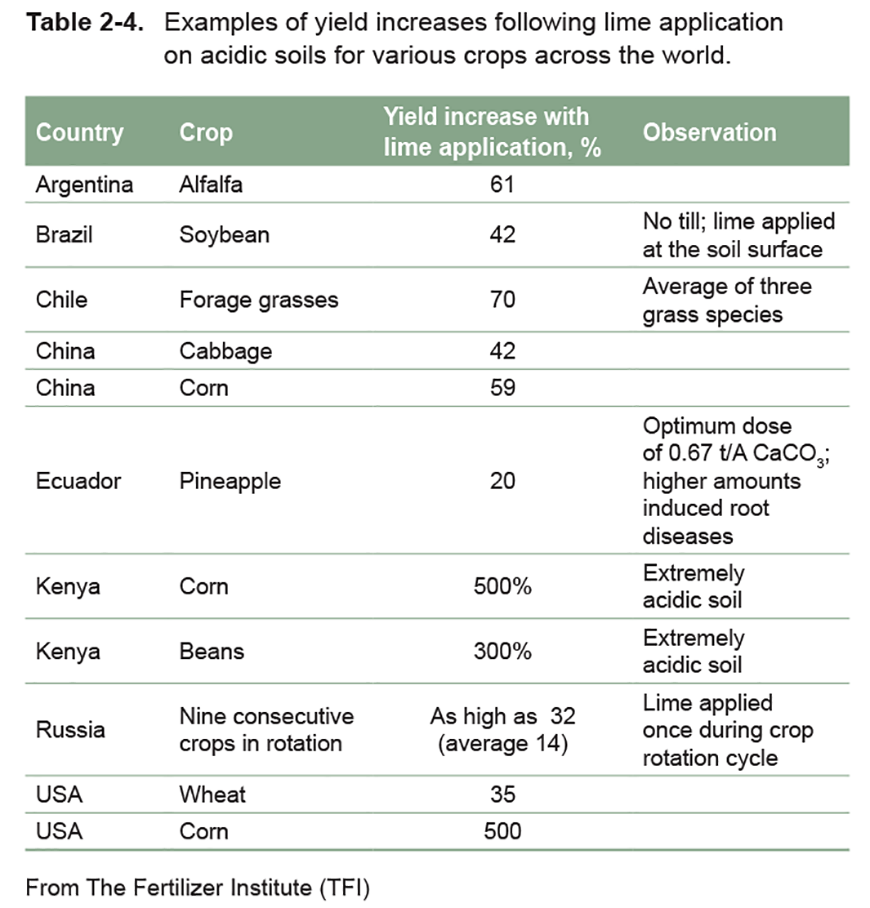
Soil Ph Nutrient Management Mosaic Crop Nutrition

17 4 Titrations And Ph Curves Chemistry Libretexts
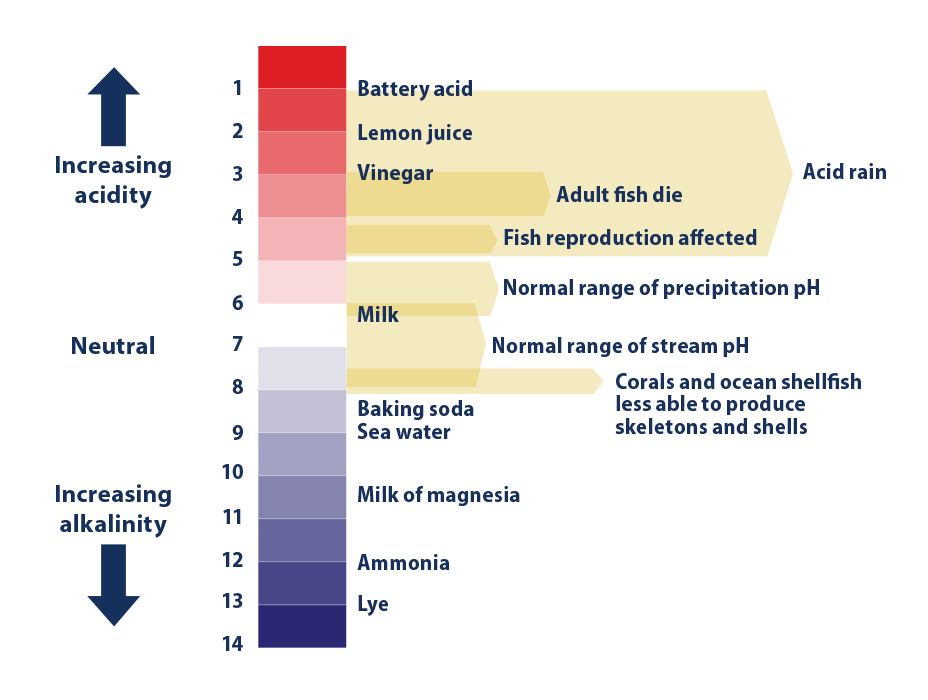
Climate Change Indicators Ocean Acidity Us Epa

Best In Glass Did You Know That Most Of The Other Beverages On The Market Use Petroleum Based Single Use Organic Superfoods Functional Beverage Lemon Health
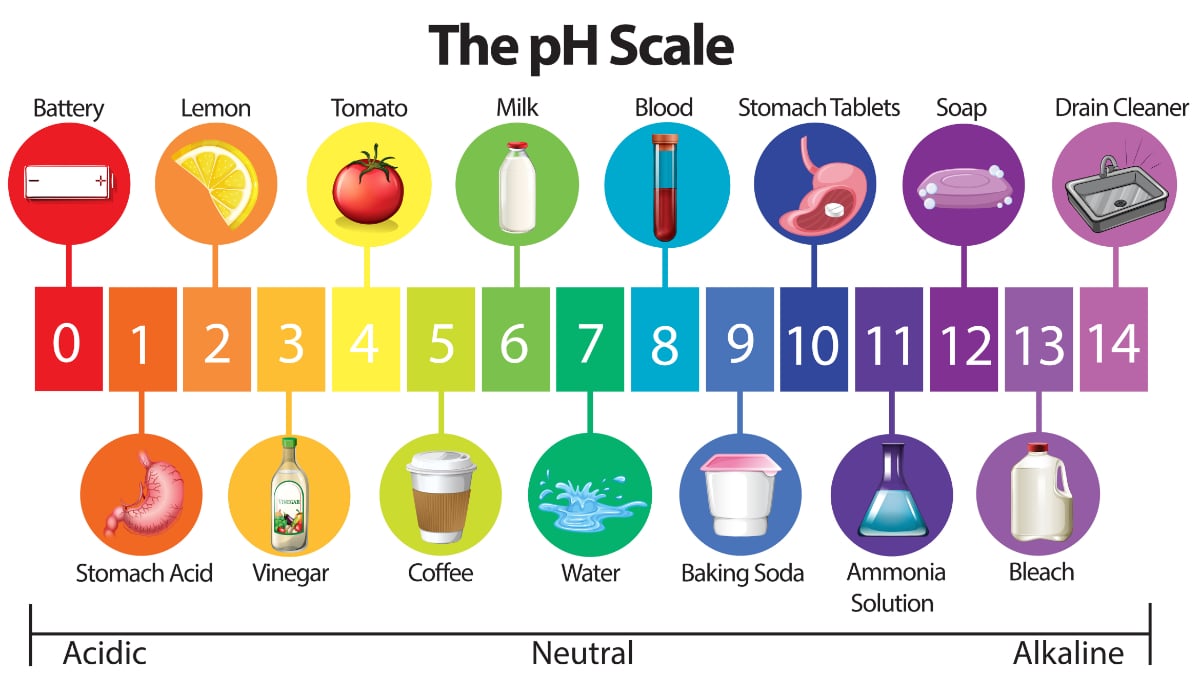
Water Quality 101 What Is Ph In Water Testing

Casio Bp 100 Relogio Casio Relogios
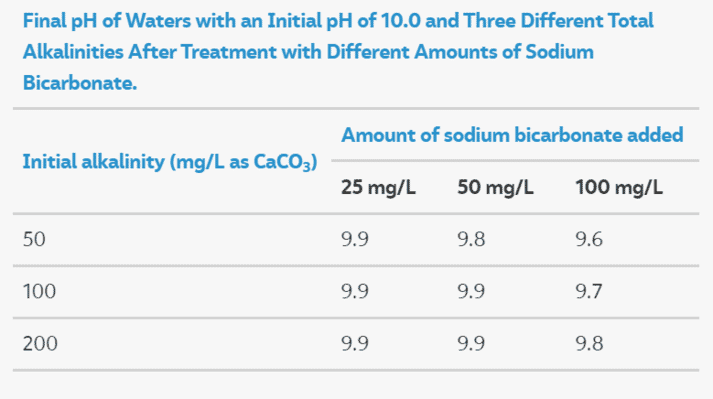
Managing High Ph In Freshwater Ponds The Fish Site

How To Freeze Kefir Grains And How To Thaw Kefir Grains Youtube Kefir Kefir Grains Fermented Drink
The Ph Scale Woods Hole Oceanographic Institution

Travel Is To Live Poem Poetry Eco Quotes About Strength And Love Travel Poems

Vintners Daughter Skincare Vitners Daughter Vitnersdaughter Luxury Skincare Lifestyle Aesthetic Luxurysk Vintners Daughter Vintner Nutritional Skincare




Comments
Post a Comment